|
Eerikäinen, Hannele
VTT Processes, Biologinkuja 7, P.O.Box 1602, FI–02044 VTT, Finland

VTT Publications 563, May 2005, 112 p. + app. 55 p.
[in English]
ISBN 951–38–6443–X
(soft back ed.)
ISBN 951–38–6444–8 (PDF edition)
Keywords: methacrylic polymer nanoparticles, preparation of drug nanoparticles, aerosol flow reactor method, drug release, solubility
properties, particle size, morphology, crystallinity, thermal properties, drug content
Abstract
Drug-containing polymer nanoparticles are submicron-sized particles consisting of drug and stabilising or functional polymer.
In this experimental study, methacrylic polymer nanoparticles with and without incorporated model drug were prepared using
a novel method, namely, aerosol flow reactor method. This method involves first preparing a solution containing the drug and
the polymer, followed by spraying the solution as nanosized droplets into a carrier gas stream, then drying the nanoparticles
in a tubular laminar flow reactor tube, and finally collecting the nanoparticles. Model polymers used in this study were Eudragit
L, Eudragit E, and Eudragit RS, which are commonly used methacrylic polymers in the pharmaceutical industry. Model drugs studied
were beclomethasone dipropionate, ketoprofen, and naproxen.
Various properties of the prepared nanoparticles were studied, such as particle size and size distribution, morphology, crystallinity,
thermal properties, drug content, and drug release. It was found that this method could be used to produce amorphous, spherical,
homogeneous matrix-type drug-polymer nanoparticles. The size of the particles was adjusted between 90 and 200 nm by the concentration
of the solution. The morphology of the particles varied as a function of the properties and composition of the starting solution.
The nanoparticles were collected as dry powders, but the stability of the powders in an amorphous form was found to be dependent
on the interactions between the drug and the polymer. When the amount of the drug in the nanoparticles was below the solubility
limit of the drug in the polymer, the amorphous nanoparticles were found to be stable and no crystallisation of the drug took
place. When the amount of the drug was larger than the solubility limit, large crystalline structures were formed due to crystallisation
of the drug. The crystallisation was also dependent on the thermal properties of the drug, as amorphous nanoparticles consisting
of a drug having a high glass transition temperature could be collected at room temperature. A low glass transition temperature
of the drug led to crystallisation of the drug at ambient conditions, when the drug amount in the nanoparticles was larger
than the solubility limit. Drug release from the nanoparticles could be modified by using polymers having specific solubility
properties.
Contents
Abstract
Preface
List of original publications
List of symbols and abbreviations
1. Introduction
2. Review
2.1 Structures of drug nanoparticles
2.2 Applications of drug nanoparticles
2.2.1 Biodegradable and non-biodegradable materials
2.2.2 Oral administration
2.3 Theory of dissolution
2.4 Amorphous drug materials and amorphous solid solutions consisting of drug and polymer
2.5 Polymer materials of the study
2.6 Methods of preparation of drug nanoparticles
3. Objective of the study
4. Experimental
4.1 Aerosol flow reactor method for the preparation of nanoparticles
4.1.1 Starting solution and atomisation
4.1.2 Solvent evaporation
4.1.3 Particle sampling and collection
4.2 Materials
4.2.1 Drug materials
4.2.2 Polymer materials
4.3 Instrumentation and characterisation
5. Results
5.1 Particle size, particle size distribution, and particle morphology (I, II, III, IV)
5.1.1 Particle size and particle size distribution as a function of solution concentration (IV)
5.1.2 Particle size and particle morphology (I, II, III)
5.2 Collection and properties of bulk nanoparticle powder (III, IV, V)
5.2.1 Collection of the nanoparticles (III, IV, V)
5.2.2 Drug release from nanoparticles containing ketoprofen (IV, V)
5.2.3 Drug release from nanoparticles containing beclomethasone dipropionate
5.2.4 Stability of the nanoparticles
6. Summary and conclusions
References
Appendices
Publications I–V
Figures and Tables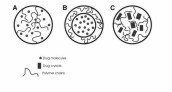 Figure 1. Schematics of exemplary types of drug nanoparticles. A. A matrix-type nanoparticle, where the drug molecules are evenly dispersed
in the polymer matrix. B. A core-shell nanoparticle, where a core containing the drug is covered with a polymer shell. C.
A matrix-type nanoparticle, where drug crystals are imbedded in a polymer matrix. Adapted from [20].
Table 1. Examples of applications and preparation methods of nanoparticles.
a) Range of mean particle sizes reported in the publication, expressed as mean±standard deviation of the distribution, where
available. Geometric standard deviation used for lognormal distributions is described in [42].
|
Application
|
Proposed administration route
|
Materials
|
Preparation method
|
Mean particle sizes reported (nm) a
|
Reference
|
|
Bioavailability increase
|
Oral
|
Avarol + PBCA
|
Emulsion polymerisation
|
136±5–707±98
|
[43]
|
|
Bioavailability increase
|
Oral
|
RR01 + Eudragit L
|
Emulsification-diffusion
|
292±22–297±6
|
[34]
|
|
Bioavailability increase
|
Oral
|
CGP 70726 + Eudragit L
|
Emulsification-diffusion
|
275±5–296±6
|
[44]
|
|
Bioavailability increase
|
Oral
|
CGP 57813 + Eudragit L / Eudragit S
|
Salting-out
|
245–264
|
[45]
|
|
Bioavailability increase
|
Oral
|
Danazol
|
Wet milling
|
169
|
[46]
|
|
Bioavailability increase
|
Oral
|
HO-221
|
Wet milling
|
453±23
|
[47]
|
|
Bioavailability increase
|
Oral
|
Buparvaquone
|
High pressure homogenisation
|
558–663
|
[48]
|
|
Bioavailability increase
|
Oral
|
Cyclosporine A
|
Evaporative precipitation into aqueous solution
|
131–526
|
[49]
|
|
Insulin administration
|
Oral
|
Insulin + PBCA
|
Interfacial emulsion polymerisation
|
136±90–152±51
|
[29]
|
|
Reduction of gastric irritation
|
Oral
|
Naproxen
|
Wet milling
|
270
|
[50]
|
|
Drug targeting
|
Ocular
|
Flurbiprofen + PCL
|
Solvent displacement
|
201–284
|
[51]
|
|
Drug targeting
|
Ocular
|
Flurbiprofen + Eudragit RS / Eudragit RL
|
Quasi-emulsion solvent diffusion
|
14–96
|
[52]
|
|
Gene delivery
|
Pulmonary
|
pDNA + PLGA-PEI
|
Solvent displacement
|
207±11–231±12
|
[53]
|
|
Sustained release
|
Pulmonary
|
Insulin + PBCA
|
Emulsion polymerisation
|
255
|
[54]
|
|
Drug delivery
|
Oral / nasal / pulmonary
|
Nafarelin acetate
+ PLGA
|
Emulsion-phase separation
|
500–800
|
[55]
|
|
Sustained release
|
i.m.
|
Savoxepine + PLA
|
Salting-out
|
230–680
|
[56]
|
|
Dissolution enhancement
|
i.v.
|
Tarazepide
|
High pressure homogenisation
|
347–517
|
[57]
|
|
Drug targeting
|
i.v.
|
Dalargin / Kyotorphin
+ PBCA
|
Emulsion polymerisation
|
195–289
|
[58]
|
|
Drug targeting
|
i.v.
|
Indomethacin /
5-fluorouracil + PLGA
|
Spontaneous emulsification solvent diffusion
|
338±67–637±40
|
[59]
|
|
Cancer therapy
|
i.v.
|
Piposulfan / Etoposide / Camptothecin / Paclitaxel
|
Wet milling
|
202±31–279±30
|
[60]
|
|
Toxicity reduction
|
i.v.
|
Primaquine + PLA
|
Solvent displacement
|
153–169
|
[61]
|
|
Drug targeting
|
No routes proposed
|
Amoxicillin
|
Supercritical antisolvent precipitation
|
250–1200
|
[62]
|
|
Drug targeting
|
No routes proposed
|
Insulin
|
Electrospray
|
88–117
|
[63]
|
|
Drug targeting
|
No routes proposed
|
Triamcinolone acetonide + PLA
|
Emulsification-evaporation
|
476±410–710±406
|
[64]
|
|
Drug targeting
|
No routes proposed
|
Atovaquone + PCL / PLA / PLGA
|
Solvent displacement
|
228±16–242±33
|
[65]
|
|
Drug targeting
|
No routes proposed
|
Tamoxifen + PCL
|
Solvent displacement
|
200–300
|
[23]
|
|
Gene delivery
|
No routes proposed
|
pDNA + PLGA
|
Double emulsion-evaporation
|
589±190–640±64
|
[7]
|
|
Sustained release
|
No routes proposed
|
Isradipine + PCL / PLA / PLGA
|
Solvent displacement
|
110–208
|
[66]
|
|
Technical studies on preparation method
|
Oral / ocular / topical
|
Indomethacin + EC / CAB / PMMA / Eudragit RS / Eudragit RL
|
Emulsification-evaporation
|
100–125
|
[12]
|
|
Technical studies on preparation method
|
No routes proposed
|
Chlorambucil + PLA / PLGA / PCL / Eudragit S
|
Emulsification-diffusion
|
246–591
|
[67]
|
|
Technical studies on preparation method
|
No routes proposed
|
PLA
|
Emulsification-diffusion
|
100–450
|
[68]
|
|
Technical studies on preparation method
|
No routes proposed
|
PAA / PMMA / PBCA / PECA / PMCA
|
Polymerisation
|
51–145
|
[16]
|
|
Technical studies on preparation method
|
No routes proposed
|
PLA / Eudragit S / Eudragit E / ethyl cellulose
|
Salting-out
|
172–1117
|
[69]
|
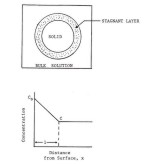 Figure 2. Schematic representation of a model depicting the dissolution process [113].  Figure 3. Diagrammatic representation of a solid carboxylic acid, HA, dissolving into a reactive medium containing hydroxide ion and buffer components B and BH+ with a Nernst diffusion layer existing between the solid and the bulk solution. Sink conditions exist in the bulk solution,
and the products, BH+, A-, and H+, diffuse out of the diffusion layer at a rate determined by their chemical reactivity and diffusivity [116].  Figure 4. Schematic depiction of the variation of enthalpy (or volume) as a function of temperature for crystalline and amorphous
(glassy) solid material [129]. 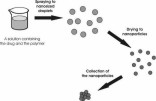 Figure 5. Schematics of particle formation in the aerosol flow reactor method. 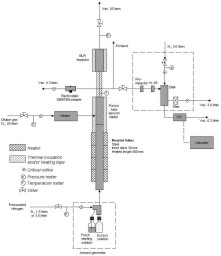 Figure 6. Experimental set-up used in the preparation of nanoparticles (N2 = clean, dry pressurized nitrogen, Vac. = vacuum, l/min = standard litres per minute, Kr-85 aerosol neutraliser using 85Kr b-source, DMA = differential mobility analyser, CPC = condensation particle counter) (II, IV, V).  Figure 7. Temperature (t (K), upper part) and velocity contours (u (m/s), lower part) in the aerosol flow reactor, 80 °C temperature, 1.5 l/min carrier gas flow rate. Courtesy of David P. Brown (published with permission) [213, 214].
Table 2. Physicochemical properties of the drug materials studied.
|
|
Beclomethasone dipropionate
|
Ketoprofen
|
Naproxen
|
|
Molecular formula
|
C28H37ClO7
|
C16H14O3
|
C14H14O3
|
|
Chemical name
|
9-Chloro-11b,17,21-trihydroxy-16b-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate
|
(2RS)-2-(3-benzoylphenol)
propanoic acid
|
(2S)-2-(6-mathoxynaphthalen-
2-yl)propanoic acid
|
|
CAS number
|
[5534-09-8]
|
[22071-15-4]
|
[22204-53-1 ]
|
|
Molecular weight (g/mol)
|
521.05
|
254.28
|
230.26
|
|
Solubility in water (mg/l)
|
150 (at 37 °C) [223]
|
118 (at 25 °C) [224]
51 (at 22 °C) [225]
|
14 (at 25 °C) [224]
16 (at 25 °C) [225]
|
|
Solubility at pH 1.2 (mg/l)
|
–
|
130 [96]
|
5 [96]
|
|
Solubility at pH 5.0 (mg/l)
|
–
|
840 [96]
|
90 [96]
|
|
Solubility at pH 7.4 (mg/l)
|
–
|
> 1400 [96]
|
> 2500 [96]
|
|
Biopharmaceutical classification system class
|
–
|
II (at pH 1.2) [96]
I (at pH 7.4) [96]
|
II (at pH 1.2) [96]
I (at pH 7.4) [96]
|
|
pKa
|
–
|
3.98 [224]
|
4.18 [224]
|
|
Melting temperature (°C)
|
212 (measured)
|
97 (measured)
|
158 (measured)
|
|
Glass transition temperature (°C)
|
66 (calculated) [125, 138]
|
-14 (calculated) [125, 138]
-2 (measured)
|
29 (calculated) [125, 138]
|
|
Particle size, 90% less than (µm)
|
6 (measured)
|
19 (measured)
|
–
|
|
Particle size, 99% less than (µm)
|
9 (measured)
|
42 (measured)
|
–
|
|
Density (g/(cm)3)
|
1.36 [226]
|
1.28 [227]
|
1.27 [228]
|
|
Molar volume ((cm3)/mol)
|
383 (calculated)
|
199 (calculated)
|
182 (calculated)
|
|
Unit cell
|
Orthorhombic [226]
|
Triclinic [227]
|
Monoclinic [228]
|
|
Unit cell dimensions
|
a = 12.12 Å [226]
b = 14.13 Å
c = 14.84 Å
a = 90 °
b = 90 °
g = 90 °
|
a = 3.89 Å [227]
b = 7.74 Å
c = 6.14 Å
a = 89.6 °
b = 94.6 °
g = 88.8 °
|
a = 13.32 Å [228]
b = 5.78 Å
c = 7.87 Å
a = 90 °
b = 93.9 °
g = 90 °
|
Table 3. Physicochemical properties of the polymer materials studied.
|
|
Eudragit L 100
|
Eudragit E 100
|
Eudragit RS 100
|
|
Composition
|
Poly(methacrylic acid, methyl methacrylate) 1:1 [161]
|
Poly(butyl methacrylate,
(2,2-dimethylaminoethyl)
methacrylate, methyl methacrylate) 1:2:1 [161]
|
Poly(ethyl acrylate,
methyl methacrylate, trimethylammoniumethyl methacrylate chloride) 1:2:0.1 [161]
|
|
Water solubility
|
Soluble at pH ³ 6 [161]
|
Soluble at pH £ 5 [161]
|
Not soluble
Not pH-dependent
|
|
Tg (°C)
|
67 (measured)
|
45 (measured)
|
64 (measured)
|
|
Density (g/(cm)3)
|
0.83-0.85 [161]
|
0.81-0.82 [161]
|
0.815-0.835 [161]
|
|
Mw (g/mol)
|
115000 [230]
|
23500 (measured)
|
39000 [231]
|
|
Polydispersity (Mw/Mn)
|
2.0 [230]
|
1.9 (measured)
|
1.5 [231]
|
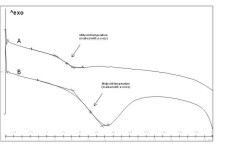 Figure 8. Determination of glass transition temperatures for exemplary curves. A) Nanoparticles containing 25% (w/w) ketoprofen /
75% (w/w) Eudragit E, B) Nanoparticles containing 0% (w/w) ketoprofen / 100% (w/w) Eudragit L.  Figure 9. Particle size distributions for the various starting solution concentrations (IV).  Figure 10. Geometric number mean diameter and calculated particle volume as a function of starting solution concentration. The dashed line represents linear
least squares fit of particle volume data points (r2 =0.99070) (IV). 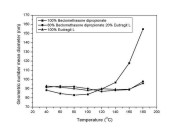 Figure 11. Geometric number mean diameter of the nanoparticles containing various amounts of drug and polymer as a function of temperature.
Total concentration of solids was 1 g/l (III).  Figure 12. TEM image of a hollow BDP nanoparticle produced (I). 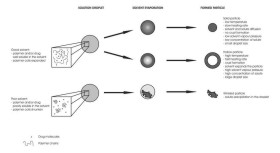 Figure 13. A summary of the effects of various parameters on particle morphology (II). 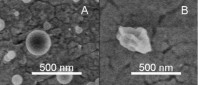 Figure 14. SEM images of polymer nanoparticles containing 50% (w/w) Eudragit L and 50% (w/w) BDP. The nanoparticles were prepared from
A) a good solvent (ethanol) or B) a mixture of a good solvent (ethanol) and a poor solvent (water). Nominal magnification
50000x.  Figure 15. X-ray diffraction patterns of the nanoparticles. A) Untreated ketoprofen. B) 50% (w/w) ketoprofen / 50% (w/w) Eudragit L
nanoparticles. C) 25% (w/w) ketoprofen / 75% (w/w) Eudragit L nanoparticles. Curve A was reduced by a factor of 4 to fit in
the same image. The curves are shifted along y-axis for clarification (IV).  Figure 16. Differential scanning calorimetry thermograms of the nanoparticles. A) Untreated ketoprofen. B) 50% (w/w) ketoprofen / 50%
(w/w) Eudragit L nanoparticles. C) 33% (w/w) ketoprofen / 67% (w/w) Eudragit L nanoparticles. D) 25% (w/w) ketoprofen / 75%
(w/w) Eudragit L nanoparticles. E) 10% (w/w) ketoprofen / 90% (w/w) Eudragit L nanoparticles. F) 100 % (w/w) Eudragit L nanoparticles.
Curve A was reduced by a factor of 20 to fit in the same image. The curves are shifted along y-axis for clarification (IV).  Figure 17. TEM image showing amorphous, spherical nanoparticles prepared. Nanoparticles containing 25% (w/w) ketoprofen and 75% (w/w)
Eudragit L. Electron optical magnification 5600x (IV).  Figure 18. Exemplary SEM images of the collected nanoparticle powders. A) Nanoparticles containing 33% (w/w) ketoprofen and 67% (w/w)
Eudragit L. Nominal magnification 50000x. B) Nanoparticles containing 10% (w/w) naproxen and 90% (w/w) Eudragit L. Nominal
magnification 50000x. C) Nanoparticles containing 67% (w/w) ketoprofen and 33% (w/w) Eudragit L. Nominal magnification 10000x.
D) Nanoparticles containing 67% (w/w) naproxen and 33% (w/w) Eudragit L. Nominal magnification 10000x (IV). 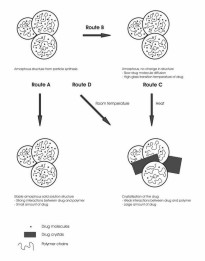 Figure 19. Schematics of the drug-polymer structures formed in particle collection.
Table 4. Glass transition temperatures of the nanoparticles prepared from ketoprofen and various polymers. a) Also an endothermic transition corresponding to the melting of ketoprofen crystals was observed at 94 °C (V).
|
Amount of ketoprofen (w/w)
|
Eudragit L
|
Eudragit E
|
Eudragit RS
|
|
0 %
|
54 °C
|
49 °C
|
53 °C
|
|
5 %
|
52 °C
|
45 °C
|
50 °C
|
|
10 %
|
50 °C
|
41 °C
|
50 °C
|
|
25 %
|
50 °C
|
24 °C
|
28 °C
|
|
33 %
|
48 °C
|
23 °C
|
20 °C
|
|
50 %
|
40 °C a)
|
–
|
–
|
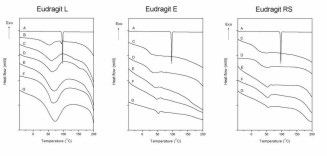 Figure 20. Differential scanning calorimetry thermograms of the ketoprofen nanoparticles. A) Untreated ketoprofen, B) Nanoparticles
containing 50% (w/w) ketoprofen, C) Nanoparticles containing 33% (w/w) ketoprofen, D) Nanoparticles containing 25% (w/w) ketoprofen,
E) Nanoparticles containing 10% (w/w) ketoprofen, F) Nanoparticles containing 5% (w/w) ketoprofen, G) Nanoparticles containing 0% (w/w) ketoprofen.
Curve A was reduced by a factor of 20 to fit in the same image. The curves are shifted along y-axis for clarification (V).  Figure 21. Exemplary scanning electron microscopy images of the ketoprofen nanoparticles. A) Nanoparticles containing 25% (w/w) ketoprofen
and 75% (w/w) Eudragit L. Nominal magnification 50000x. B) Nanoparticles containing 10% (w/w) ketoprofen and 90% (w/w) Eudragit
RS. Nominal magnification 50000x. C) Nanoparticles containing 25% (w/w) ketoprofen and 75% (w/w) Eudragit E. Nominal magnification
5000x. D) Nanoparticles containing 25% (w/w) ketoprofen and 75% (w/w) Eudragit RS. Nominal magnification 10000x ().  Figure 22. Glass transition temperatures of the nanoparticles containing ketoprofen and polymer. For the calculated values, the glass
transition temperature of the nanoparticles consisting of only polymer was used as the polymer glass transition temperature. 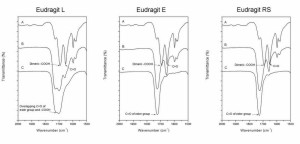 Figure 23. Exemplary infrared spectra at a wavenumber range of 2000–1500 cm-1. A) Pure ketoprofen. B) Nanoparticles containing 33% (w/w) ketoprofen. C) Pure polymer. The curves are shifted along y-axis
for clarification (V).  Figure 24. Exemplary infrared spectra at a wavenumber range of 3500–2500 cm-1. A) Pure ketoprofen. B) Nanoparticles containing 33% (w/w) ketoprofen. C) Pure polymer. The curves are shifted along y-axis
for clarification (V).  Figure 25. DSC scans of nanoparticles containing various amounts of BDP and Eudragit L. A) 100% (w/w) BDP, B) 80% (w/w) BDP / 20% (w/w)
Eudragit L, C) 60% (w/w) BDP / 40% (w/w) Eudragit L, D) 50% (w/w) BDP / 50% (w/w) Eudragit L, E) 100% (w/w) Eudragit L. Observed
crystallisation and melting of the crystals are marked with cr and m, respectively . The curves are shifted along y-axis for clarification (III).  Figure 26. Ketoprofen release for nanoparticles containing various amounts of drug as a function of time (IV). Pure ketoprofen denotes untreated, commercial, crystalline ketoprofen powder (particle size 90% less than 19 µm).  Figure 27. Ketoprofen release for nanoparticles containing various polymers as a function of time. All the nanoparticles studied for
drug release contained 10% (w/w) ketoprofen (V). Pure ketoprofen denotes untreated, commercial, crystalline ketoprofen powder (particle size 90% less than 19 µm). 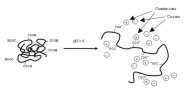 Figure 28. Schematic representation of dissolution of a polyelectrolyte containing carboxylic acid groups (Eudragit L).  Figure 29. Compartmental absorption and transit model [264].  Figure 30. CAT modeling (CAT predicted) of literature reference data (Observed) and CAT modeling (Nanoparticles predicted) of nanoparticles
containing ketoprofen and Eudragit RS.  Figure 31. BDP release for pure BDP (particle size 90% less than 6 µm) and BDP nanoparticles.  Figure 32. X-ray diffraction patterns of nanoparticle dry powder containing 33% (w/w) ketoprofen and 67% (w/w) Eudragit L after 3 months
storage time. A) Nanoparticle dry powder stored in a refrigerator. B) Nanoparticle dry powder stored at 25 °C at 0% relative humidity. C) Nanoparticle dry powder stored at 25 °C at 75% relative humidity. The curves are shifted along y-axis for clarification.
References
[1] Allémann, E., Gurny, R., and Doelker, E. Drug-loaded nanoparticles - preparation methods and drug targeting issues. European
Journal of Pharmaceutics and Biopharmaceutics, 1993. Vol. 39, pp. 173–191.
[2] Kawashima, Y. Nanoparticulate systems for improved drug delivery. Advanced Drug Delivery Reviews, 2001. Vol. 47, pp. 1–2.
[3] Soppimath, K. S., Aminabhavi, T. M., Kulkarni, A. R., and Rudzinski, W. E. Biodegradable polymeric nanoparticles as drug
delivery devices. Journal of Controlled Release, 2001. Vol. 70, pp. 1–20.
[4] Panyam, J. and Labhasetwar, V. Biodegradable nanoparticle from drug and gene delivery to cells and tissue. Advanced Drug
Delivery Reviews, 2003. Vol. 55, pp. 329–347.
[5]´Brigger, I., Dubernet, C., and Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Advanced Drug Delivery Reviews,
2002. Vol. 54, pp. 631–651.
[6] Cui, Z. and Mumper, R. J. Plasmid DNA-entrapped nanoparticles engineered from microemulsion precursors: In vitro and in
vivo evaluation. Bioconjugate Chemistry, 2002. Vol. 13, pp. 1319–1327.
[7] Cohen, H., Levy, R. J., Gao, J., Fishbein, I., Kousaev, V., Sosnowski, S., Slomkowski, S., and Golomb, G. Sustained delivery
and expression of DNA encapsulated in polymeric nanoparticles. Gene Therapy, 2000. Vol. 7, pp. 1896–1905.
[8] Kreuter, J. Nanoparticles and Nanocapsules – New Dosage Forms in the Nanometer Range. Pharmaceutica Acta Helvetiae, 1978.
Vol. 53, pp. 33–39.
[9] Kreuter, J. Nanoparticles. In: Swarbrick, J. and Boylan, J. C. (Eds.) Encyclopedia of Pharmaceutical Technology. pp. 165–190.
New York, USA: Marcel Dekker, Inc., 1994. ISBN 0-8247-2809-2
[10] Birrenbach, G. and Speiser, P. P. Polymerized micelles and their use as adjuvants in immunology. Journal of Pharmaceutical
Sciences, 1976. Vol. 65, pp. 1763–1766.
[11] Couvreur, P., Dubernet, C., and Puisieux, F. Controlled drug delivery with nanoparticles: current possibilities and future
trends. European Journal of Pharmaceutics and Biopharmaceutics, 1995. Vol. 41, pp. 2–13.
[12] Bodmeier, R. and Chen, H. Indomethacin polymeric nanosuspensions prepared by microfluidization. Journal of Controlled
Release, 1990. Vol. 12, pp. 223–233.
[13] de Chasteigner, S., Fessi, H., Cavé, G., Devissaguet, J. P., and Puisieux, F. Gastro-intestinal tolerance study of a
freeze-dried oral dosage form of indomethacin-loaded nanocapsules. STP Pharma Sciences, 1995. Vol. 5, pp. 242–246.
[14] Pohlmann, A. R., Weiss, V., Mertins, O., Pesce da Silveira, N., and Guterres, S. S. Spray-dried indomethacin-loaded polyester
nanocapsules and nanospheres: development, stability evaluation and nanostructure models. European Journal of Pharmaceutical
Sciences, 2002. Vol. 16, pp. 305–312.
[15] Saez, A., Guzman, M., Molpeceres, J., and Aberturas, M. R. Freeze-drying of polycaprolactone and poly(-lactic-glycolic)
nanoparticles induce minor particle size changes affecting the oral pharmacokinetics of loaded drugs. European Journal of
Pharmaceutics and Biopharmaceutics, 2000. Vol. 50, pp. 379–387.
[16] Kreuter, J. Physicochemical characterization of polyacrylic nanoparticles. International Journal of Pharmaceutics, 1983.
Vol. 14, pp. 43–58.
[17] Legrand, P., Barratt, G., Mosqueira, V., Fessi, H., and Devissaguet, J. P. Polymeric nanocapsules as drug delivery systems.
A review. STP Pharma Sciences, 1999. Vol. 9, pp. 411–418.
[18] Barratt, G. M. Therapeutic applications of colloidal drug carriers. Pharmaceutical Science and Technology Today, 2000.
Vol. 3, pp. 163–171.
[19] Fresta, M., Cavallaro, G., Giammona, G., Wehrli, E., and Puglisi, G. Preparation and characterization of polyethyl-2-cyanoacrylate
nanocapsules containing antiepileptic drugs. Biomaterials, 1996. Vol. 17, pp. 751–758.
[20] Vauthier-Holtzscherer, C., Benabbou, S., Spenlehauer, G., Veillard, M., and Couvreur, P. Methodology for the preparation
of ultra-dispersed polymer systems. STP Pharma Sciences, 1991. Vol. 1, pp. 109–116.
[21] Brannon-Peppas, L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery.
International Journal of Pharmaceutics, 1995. Vol. 116, pp. 1–9.
[22] Schmidt, C. and Bodmeier, R. Incorporation of polymeric nanoparticles into solid dosage forms. Journal of Controlled
Release, 1999. Vol. 57, pp. 115–125.
[23] Chawla, J. S. and Amiji, M. M. Biodegradable poly(e-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. International Journal of Pharmaceutics, 2002. Vol.
249, pp. 127–138.
[24] Labhasetwar, V., Song, C., and Levy, R. J. Nanoparticle drug delivery system for restenosis. Advanced Drug Delivery Reviews,
1997. Vol. 24, pp. 63–85.
[25] Yang, L. and Alexandritis, P. Physicochemical aspects of drug delivery and release from polymer-based colloids. Current
Opinion in Colloid & Interface Science, 2000. Vol. 5, pp. 132–143.
[26] Vauthier, C., Dubernet, C., Fattal, E., Pinto-Alphandary, H., and Couvreur, P. Poly(alkylcyanoacrylates) as biodegradable
materials for biomedical applications. Advanced Drug Delivery Reviews, 2003. Vol. 55, pp. 519–548.
[27] Lambert, G., Fattal, E., and Couvreur, P. Nanoparticulate systems for the delivery of antisense oligonucleotides. Advanced
Drug Delivery Reviews, 2001. Vol. 47, pp. 99–112.
[28] Damgé, C., Michel, C., Aprahamian, M., Couvreur, P., and Devissaguet, J. P. Nanocapsules as carriers for oral peptide
delivery. Journal of Controlled Release, 1990. Vol. 13, pp. 233–239.
[29] Damgé, C., Vranckx, H., Balschmidt, P., and Couvreur, P. Poly(alkyl cyanoacrylate) nanospheres for oral administration
of insulin. Journal of Pharmaceutical Sciences, 1997. Vol. 86, pp. 1403–1409.
[30] Gurny, R., Boye, T., and Ibrahim, H. Ocular therapy with nanoparticulate systems for controlled drug delivery. Journal
of Controlled Release, 1985. Vol. 2, pp. 353–361.
[31] Khopade, A. J. and Jain, N. K. Self assembling nanostructures for sustained ophthalmic drug delivery. Pharmazie, 1995.
Vol. 50, pp. 812–814.
[32] Pignatello, R., Bucolo, C., Ferrara, P., Maltese, A., Puleo, A., and Puglisi, G. Eudragit RS100 nanosuspensions for the
ophthalmic controlled delivery of ibuprofen. European Journal of Pharmaceutical Sciences, 2002. Vol. 16, pp. 53–61.
[33] de Vringer, T. and de Ronde, H. A. G. Preparation and structure of a water-in-oil cream containing lipid nanoparticles.
Journal of Pharmaceutical Sciences, 1995. Vol. 84, pp. 466–472.
[34] De Jaeghere, F., Allémann, E., Doelker, E., and Gurny, R. pH-dependent dissolving nano- and microparticles for improved
peroral delivery of a highly lipophilic compound in dogs. AAPS Pharmsci, 2001. Vol. 3, pp. Article 8.
[35] Kreuter, J. Peroral administration of nanoparticles. Advanced Drug Delivery Reviews, 1991. Vol. 7, pp. 71–86.
[36] Müller, R. H., Jacobs, C., and Kayser, O. Nanosuspensions as particulate drug formulations in therapy Rationale for development
and what can we expect for the future. Advanced Drug Delivery Reviews, 2001. Vol. 47, pp. 3–19.
[37] Shekunov, B. Y. and York, P. Crystallization processes in pharmaceutical technology and drug delivery design. Journal
of Crystal Growth, 2000. Vol. 211, pp. 122–136.
[38] Mehnert, W. and Mäder, K. Solid lipid nanoparticles Production, characterization and applications. Advanced Drug Delivery
Reviews, 2001. Vol. 47, pp. 165–196.
[39] Miller, K. J. and Das, S. K. Antisense oligonucleotides: strategies for delivery. Pharmaceutical Science and Technology
Today, 1998. Vol. 1, pp. 377–386.
[40] Hans, M. L. and Lowman, A. M. Biodegradable nanoparticles for drug delivery and targeting. Current Opinion in Solid State
and Materials Science, 2002. Vol. 6, pp. 319–327.
[41] Moghimi, S. M., Hunter, A. C., and Murray, J. C. Long-circulating and target-specific nanoparticles: theory to practice.
Pharmacological Reviews, 2001. Vol. 53, pp. 283–318.
[42] Hinds, W. C. Aerosol technology : properties, behavior, and measurement of airborne particles. New York, USA: John Wiley
& Sons, Inc., 1999. ISBN 0-471-19410-7.
[43] Beck, P. H., Kreuter, J., Müller, W. E. G., and Schatton, W. Improved peroral delivery of avarol with polybutylcyanoacrylate
nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics, 1994. Vol. 40, pp. 134–137.
[44] De Jaeghere, F., Allémann, E., Kubel, F., Galli, B., Cozens, R., Doelker, E., and Gurny, R. Oral bioavailability of a
poorly water soluble HIV-1 protease inhibitor incorporated into pH-sensitive particles: effect of the particle size and nutritional
state. Journal of Controlled Release, 2000. Vol. 68, pp. 291–298.
[45] Leroux, J.-C., Cozens, R. M., Roesel, J. L., Galli, B., Doelker, E., and Gurny, R. pH-sensitive nanoparticles: an effective
means to improve the oral delivery of HIV-1 protease inhibitors in dogs. Pharmaceutical Research, 1996. Vol. 13, pp. 485–487.
[46] Liversidge, G. G. and Cundy, K. C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs:
I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. International Journal of Pharmaceutics, 1995.
Vol. 125, pp. 91–97.
[47] Kondo, N., Iwao, T., Masuda, H., Yamanouchi, K., Ishihara, Y., Yamada, N., Haga, T., Ogawa, Y., and Yokoyama, K. Improved
oral absorption of a poorly water-soluble drug, HO-221, by wet-bead milling producing particles in a submicron region. Chemical
and Pharmaceutical Bulletin, 1993. Vol. 41, pp. 737–740.
[48] Müller, R. H. and Jacobs, C. Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability.
International Journal of Pharmaceutics, 2002. Vol. 237, pp. 151–161.
[49] Chen, X., Young, T. J., Sarkari, M., Williams III, R. O., and Johnston, K. P. Preparation of cyclosporine A nanoparticles
by evaporative precipitation into aqueous solution. International Journal of Pharmaceutics, 2002. Vol. 242, pp. 3–14.
[50] Liversidge, G. G. and Conzentino, P. Drug particle size reduction for decreasing gastric irritancy and enhancing absorption
of naproxen in rates. International Journal of Pharmaceutics, 1995. Vol. 125, pp. 309–313.
[51] Gamisans, F., Lacoulonche, F., Chauvet, A., Espina, M., Garcia, M. L., and Egea, M. A. Flurbiprofen-loaded nanospheres:
analysis of the matrix structure by thermal methods. International Journal of Pharmaceutics, 1999. Vol. 179, pp. 37–48.
[52] Pignatello, R., Bucolo, C., Spedalieri, G., Maltese, A., and Puglisi, G. Flurbiprofen-loaded acrylate polymer nanosuspensions
for ophthalmic application. Biomaterials, 2002. Vol. 23, pp. 3247–3255.
[53] Bival-Benita, M., Romeijn, S., Juninger, H. E., and Borchard, G. PLGA-PEI nanoparticles for gene delivery to pulmonary
epithelium. European Journal of Pharmaceutics and Biopharmaceutics, 2004. Vol. 58, pp. 1–6.
[54] Zhang, Q., Shen, Z., and Nagai, T. Prolonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticle
after pulmonary administration to normal rats. International Journal of Pharmaceutics, 2001. Vol. 218, pp. 75–80.
[55] Niwa, T., Takeuchi, H., Hino, T., Nohara, M., and Kawashima, Y. Biodegradable submicron carriers for peptide drugs: Preparation
of DL-lactide/glycolide copolymer (PLGA) nanospheres with nafarelin acetate by a novel emulsion-phase separation method in
an oil system. International Journal of Pharmaceutics, 1995. Vol. 121, pp. 45–54.
[56] Allémann, E., Doelker, E., and Gurny, R. Drug loaded poly(lactic acid) nanoparticles produced by a reversible salting-out
process: purification of an injectable dosage form. European Journal of Pharmaceutics and Biopharmaceutics, 1993. Vol. 39,
pp. 13–18.
[57] Jacobs, C., Kayser, O., and Müller, R. H. Nanosuspensions as a new approach for the formulation for the poorly soluble
drug tarazepide. International Journal of Pharmaceutics, 2000. Vol. 196, pp. 161–164.
[58] Schroeder, U., Sommerfeld, P., Ulrich, S., and Sabel, B. A. Nanoparticle technology for delivery of drugs across the
Blood-Brain Barrier. Journal of Pharmaceutical Sciences, 1998. Vol. 87, pp. 1305–1307.
[59] Niwa, T., Takeuchi, H., Hino, T., Kunou, N., and Kawashima, Y. Preparations of biodegradable nanospheres of water-soluble
and insoluble drugs with D,L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and
the drug release behaviour. Journal of Controlled Release, 1993. Vol. 25, pp. 89–98.
[60] Merisko-Liversidge, E., Sarpotdar, P., Bruno, J., Hajj, S., Wei, L., Peltier, N., Rake, J., Shaw, J. M., Pugh, S., Polin,
L., Jones, J., Corbett, T., Cooper, E., and Liversidge, G. G. Formulation and antitumor activity evaluation of nanocrystalline
suspensions of poorly soluble anticancer drugs. Pharmaceutical Research, 1996. Vol. 13, pp. 272–278.
[61] Rodrigues Jr., J. M., Fessi, H., Bories, C., Puisieux, F., and Devissaguet, J.-P. Primaquine-loaded poly(lactide) nanoparticles:
physicochemical study and acute tolerance in mice. International Journal of Pharmaceutics, 1995. Vol. 126, pp. 253–260.
[62] Reverchon, E., Della Porta, G., and Falivene, M. G. Process parameters and morphology in amoxicillin micro and submicro
particles generation by supercritical antisolvent precipitation. Journal of Supercritical Fluids, 2000. Vol. 17, pp. 239–248.
[63] Gomez, A., Bingham, D., de Juan, L., and Tang, K. Production of protein nanoparticles by electrospray drying. Journal
of Aerosol Science, 1998. Vol. 29, pp. 561–574.
[64] Krause, H.-J., Schwarz, A., and Rohdewald, P. Polylactic acid nanoparticles, a colloidal delivery system for lipophilic
drugs. International Journal of Pharmaceutics, 1985. Vol. 27, pp. 145–155.
[65] Cauchetier, E., Deniau, M., Fessi, H., Astier, A., and Paul, M. Atovaquone-loaded nanocapsules: influence of the nature
of the polymer on their in vitro characteristics. International Journal of Pharmaceutics, 2003. Vol. 250, pp. 273–281.
[66] Leroueil-Le Verger, M., Fluckiger, L., Kim, Y.-I., Hoffman, M., and Maincent, P. Preparation and characterization of
nanoparticles containing an antihypertensive agent. European Journal of Pharmaceutics and Biopharmaceutics, 1998. Vol. 46,
pp. 137–143.
[67] Leroux, J.-C., Allémann, E., Doelker, E., and Gurny, R. New approach for the preparation of nanoparticles by an emulsification-diffusion
method. European Journal of Pharmaceutics and Biopharmaceutics, 1995. Vol. 41, pp. 14–18.
[68] Quintanar-Guerrero, D., Fessi, H., Allémann, E., and Doelker, E. Influence of stabilizing agents and preparative variables
on the formation of poly(D,L-lactic acid) nanoparticles by an emulsification-diffusion technique. International Journal of
Pharmaceutics, 1996. Vol. 143, pp. 133–141.
[69] Allémann, E., Gurny, R., and Doelker, E. Preparation of aqueous polymeric nanodispersions by a reversible salting-out
process: influence of process parameters on particle size. International Journal of Pharmaceutics, 1992. Vol. 87, pp. 247–253.
[70] Leroux, J.-C., Cozens, R., Roesel, J. L., Galli, B., Kubel, F., Doelker, E., and Gurny, R. Pharmacokinetics of a novel
HIV-1 protease inhibitor incorporated into biodegradable or enteric nanoparticles following intravenous and oral administration
to mice. Journal of Pharmaceutical Sciences, 1995. Vol. 84, pp. 1387–1391.
[71] Jacobs, C., Kayser, O., and Müller, R. H. Production and characterisation of mucoadhesive nanosuspensions for the formulation
of bupravaquone. International Journal of Pharmaceutics, 2001. Vol. 214, pp. 3–7.
[72] Merisko-Liversidge, E., Liversidge, G. G., and Cooper, E. R. Nanosizing: a formulation approach for poorly-water-soluble
compounds. European Journal of Pharmaceutical Sciences, 2003. Vol. 18, pp. 113–120.
[73] Mitchell, S. A., Reynolds, T. D., and Dasbach, T. P. A compaction process to enhance dissolution of poorly water-soluble
drugs using hydroxypropyl methylcellulose. International Journal of Pharmaceutics, 2003. Vol. 250, pp. 3–11.
[74] Müller, R. H., Schmidt, S., Buttle, I., Akkar, A., Schmitt, J., and Brömer, S. SolEmuls-novel technology for the formulation
of i.v. emulsions with poorly soluble drugs. International Journal of Pharmaceutics, 2004. Vol. 269, pp. 293–302.
[75] Müller, R. H. and Peters, K. Nanosuspensions for the formulation of poorly soluble drugs I. Preparation by a size-reduction
technique. International Journal of Pharmaceutics, 1998. Vol. 160, pp. 229–237.
[76] Pillay, V. and Fassihi, R. Unconventional dissolution methologies. Journal of Pharmaceutical Sciences, 1999. Vol. 88,
pp. 843–851.
[77] Vadnere, M. K. Coprecipitates and melts. In: Swarbrick, J. and Boylan,
J. C. (Eds.) Encyclopedia of Pharmaceutical Technology. Pp. 337–352. New York, USA: Marcel Dekker, Inc., 1990. ISBN 0-8247-2802-5.
[78] Humberstone, A. J. and Charman, W. N. Lipid-based vehicles of the oral delivery of poorly water-soluble drugs. Advanced
Drug Delivery Reviews, 1997. Vol. 25, pp. 103–128.
[79] Vemuri, S. and Rhodes, C. T. Preparation and characterization of liposomes as therapeutic delivery systems: a review.
Pharmaceutica Acta Helvetiae, 1995. Vol. 70, pp. 95–111.
[80] Duchêne, D., Wouessidjewe, D., and Ponchel, G. Cyclodextrins and carrier systems. Journal of Controlled Release, 1999.
Vol. 62, pp. 263–268.
[81] Hirayama, F. and Uekama, K. Cyclodextrin-based controlled drug release system. Advanced Drug Delivery Reviews, 1999.
Vol. 36, pp. 125–141.
[82] Loftsson, T. and Brewster, M. E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization.
Journal of Pharmaceutical Sciences, 1996. Vol. 85, pp. 1017–1025.
[83] Beten, D. B. and Moës, A. J. Controlled-release coevaporates of dipyridamole prepared wth acrylic polymers. International
Journal of Pharmaceutics, 1994. Vol. 103, pp. 243–251.
[84] Ford, J. L. The current status of solid dispersions. Pharmaceutica Acta Helvetiae, 1986. Vol. 61, pp. 69–88.
[85] Sarkari, M., Brown, J., Chen, X., Swinnea, S., Williams III, R. O., and Johnston, K. P. Enhanced drug dissolution using
evaporative precipitation into aqueous solution. International Journal of Pharmaceutics, 2002. Vol. 243,
pp. 17–31.
[86] Corrigan, O. I., Holohan, E. M., and Sabra, K. Amorphous forms of thiazide diuretics prepared by spray-drying. International
Journal of Pharmaceutics, 1984. Vol. 18, pp. 195–200.
[87] Kondo, N., Iwao, T., Kikuchi, M., Shu, H., Yamanouchi, K., Yokoyama, K., Obyama, K., and Ogyu, S. Pharmacokinetics of
a micronized, poorly water-soluble drug, HO-221, in experimental animals. Biological and Pharmaceutical Bulletin, 1993. Vol.
16, pp. 796–800.
[88] Nimmerfall, F. and Rosenthaler, J. Dependence of area under the curve on proquazone particle size and in vitro dissolution rate. Journal of Pharmaceutical Sciences, 1980. Vol. 69, pp. 605–607.
[89] Mosharraf, M. and Nyström, C. The effect of particle size and shape on the surface specific dissolution rate of microsized
practically insoluble drugs. International Journal of Pharmaceutics, 1995. Vol. 122, pp. 35–47.
[90] Hörter, D. and Dressman, J. B. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal
tract. Advanced Drug Delivery Reviews, 1997. Vol. 25, pp. 3–14.
[91] Florence, A. T. and Attwood, D. Physicochemical Principles of Pharmacy. Hampshire and London, UK: The Macmillan Press
Ltd., 1988. ISBN 0-333-44996-7
[92] Hussain, A. S., Lesko, L. J., Lo, K. Y., Shah, V. P., Volpe, D., and Williams, B. L. The biopharmaceutics classification
system: highlights of the FDA's draft guidance. Dissolution Technologies, 1999. Vol. 6, pp. 5–9.
[93] Yu, L. X., Amidon, G. L., Polli, J. E., Zhao, H., Mehta, M. U., Conner, D. P., Shah, V. P., Lesko, L. J., Chen, M.-L.,
Lee, V. H. L., and Hussain, A. S. Biopharmaceutics classification system: the scientific basis for biowaiver extensions. Pharmaceutical
Research, 2002. Vol. 19, pp. 921–925.
[94] Dressman, J., Butler, J., Hempenstall, J., and Reppas, C. The BCS: where do we from here. Pharmaceutical Technology,
2001. Vol. July, pp. 68–76.
[95] Amidon, G. L., Lennernäs, H., Shah, V. P., and Crison, J. R. A theoretical basis for a biopharmaceutic drug classification:
the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharmaceutical Research, 1995. Vol. 12, pp. 413–420.
[96] Yazdanian, M., Briggs, K., Jankovsky, C., and Hawi, A. The "high solubility" definition of the current FDA guidance on
biopharmaceutical classification system may be too strict for acidic drugs. Pharmaceutical Research, 2004. Vol. 21, pp. 293–299.
[97] Vandamme, T. F., Lenourry, A., Charrueau, C., and Chaumeil, J.-C. The use of polysaccharides to target drugs to the colon.
Carbohydrate polymers, 2002. Vol. 48, pp. 219–231.
[98] Chambliss, W. G. Enteric Coatings. In: Swarbrick J. and Boylan, J. C. (Eds.) Encyclopedia of Pharmaceutical Technology.
Pp. 189–200. New York, USA: Marcel Dekker, Inc., 1992. ISBN 0-8247-2804-1.
[99] Seitz, J. A., Mehta, S. P., and Yeager, J. L. Tablet Coating. In: Lachman, L., Lieberman H. A. and Kanig, J. L. (Eds.)
The Theory and Practice of Industrial Pharmacy. Pp. 346–373. Philadelphia, USA: Lea & Febiger, 1986. ISBN 0-8121-0977-5.
[100] Earnest, D. L. NSAID-induced gastric injury: Its pathogenesis and management. Seminars in Arthritis and Rheumatism,
1990. Vol. 19, pp. 6–10.
[101] Fara, J. W. and Myrback, R. E. Formulation and dosage form design in drug-induced topical irritation of the gastrointestinal
tract. Pharmaceutical Research, 1990. Vol. 7, pp. 616–620.
[102] Palmieri, G. F., Bonacucina, G., Di Martino, P., and Martelli, S. Microencapsulation of semisolid ketoprofen/polymer
microspheres. International Journal of Pharmaceutics, 2002. Vol. 242, pp. 175–178.
[103] Cioli, V., Putzolu, S., Rossi, V., Scorza Barcellona, P., and Corradino, C. The role of direct tissue contact in the
production of gastrointestinal ulcers by anti-inflammatory drugs in rates. Toxicology and Applied Pharmacology, 1979. Vol.
50, pp. 283–289.
[104] Ammoury, N., Fessi, H., Devissaguet, J.-P., Dubrasquet, M., and Benita, S. Jejunal absorption, pharmacological activity,
and pharmacokinetic evaluation of indomethacin-loaded poly(D,L-lactide) and poly(isobutyl-cyanoacrylate) nanocapsules in rats.
Pharmaceutical Research, 1991. Vol. 8, pp. 101–105.
[105] Guterres, S. S., Fessi, H., Barratt, G., Puisieux, F., and Devissaguet, J.-P. Poly(D,L-lactide) nanocapsules containing
non-steroidal anti-inflammatory drugs: gastrointestinal tolerance following intravenous and oral administration. Pharmaceutical
Research, 1995. Vol. 12, pp. 1545–1547.
[106] Kondo, N., Iwao, T., Hirai, K.-I., Fukuda, M., Yamanouchi, K., Yokoyama, K., Miyaji, M., Ishihara, Y., Kon, K., Ogawa,
Y., and Mayumi, T. Improved oral absorption of enteric coprecipitates of a poorly soluble drug. Journal of Pharmaceutical
Sciences, 1994. Vol. 83, pp. 566–570.
[107] Andrianov, A. K. and Payne, L. G. Polymeric carriers for oral uptake of microparticulates. Advanced Drug Delivery Reviews,
1998. Vol. 34, pp. 155–170.
[108] Couvreur, P. and Puisieux, F. Nano- and microparticles for the delivery of polypeptides and proteins. Advanced Drug
Delivery Reviews, 1993. Vol. 10,
pp. 141–162.
[109] Morishita, M., Morishita, I., Takayama, K., Machida, Y., and Nagai, T. Novel oral microspheres of insulin with protease
inhibitor protecting from enzymatic degradation. International Journal of Pharmaceutics, 1992. Vol. 78, pp. 1–7.
[110] Bruner, L. and Tolloczko, S. Über die Auflösungsgeschwindigkeit Fester Körper. Zeitscrift für Physikalische Chemie,
1900. Vol. 35, pp. 283–290.
[111] Nernst, W. Theorie der Reaktionsgeschwindigkeit in Heterogenen Systemen. Zeitscrift für Physikalische Chemie, 1904.
Vol. 47, pp. 52–55.
[112] Brunner, E. Reaktionsgeschwindigkeit in Heterogenen Systemen. Zeitscrift für Physikalische Chemie, 1904. Vol. 47, pp.
56–102.
[113] Banakar, U. V. Pharmaceutical Dissolution Testing. New York, USA: Marcel Dekker, Inc., 1992. ISBN 0-8247-8567-3.
[114] Abdou, H. M. Dissolution, Bioavailability & Bioequivalence. Easton, USA: Mack Printing Company, 1989. ISBN 0-912-734-20-5
[115] Mooney, K. G., Mintun, M. A., Himmelstein, K. J., and Stella, V. J. Dissolution kinetics of carboxylic acids I: Effect
of pH under unbuffered conditions. Journal of Pharmaceutical Sciences, 1981. Vol. 70, pp. 13–22.
[116] Mooney, K. G., Mintun, M. A., Himmelstein, K. J., and Stella, V. J. Dissolution kinetics of carboxylic acids II: Effect
of buffers. Journal of Pharmaceutical Sciences, 1981. Vol. 70, pp. 22–32.
[117] Serajuddin, A. T. M. and Jarowski, C. I. Effect of diffusion layer pH and solubility on the dissolution rate of pharmaceutical
bases and their hydrochloride salts I: Phenazopyridine. Journal of Pharmaceutical Sciences, 1985. Vol. 74, pp. 142–147.
[118] Serajuddin, A. T. M. and Jarowski, C. I. Effect of diffusion layer pH and solubility on the dissolution rate of pharmaceutical
acids and their sodium salts II: Salicylic acid, theophylline, and benzoic acid. Journal of Pharmaceutical Sciences, 1985.
Vol. 74, pp. 148–154.
[119] Higuchi, W. I., Parrott, E. L., Wurster, D. E., and Higuchi, T. Investigation of drug release from solids II. Theoretical
and experimental study of influences of bases and buffers on rates of dissolution of acidic solids. Journal of the American
Pharmaceutical Association, 1958. Vol. 47, pp. 376–383.
[120] Buckton, G. Interfacial Phenomena in Drug Delivery and Targeting. In: Florence, A. T. and Gregoriadis, G. (Eds.) Drug
Delivery and Targeting. Chur, Switzerland: Harwood Academic Publishers, 1995. ISBN 3-7186-5633-7.
[121] Noyes, A. A. and Whitney, W. R. Ueber die Auflösungsgeschwindigkeit von Festen Stoffen in Ihren Eigenen Lösungen. Zeitscrift
für Physikalische Chemie, 1897. Vol. 23, pp. 689–692.
[122] Vergote, G. J., Vervaet, C., Van Driessche, I., Hoste, S., De Smedt, S., Demeester, J., Jain, R. A., Ruddy, S., and
Remon, J. P. In vivo evaluation of matrix pellets containing nanocrystalline ketoprofen. International Journal of Pharmaceutics,
2002. Vol. 240, pp. 79–84.
[123] Buckton, G. and Darcy, P. Assessment of disorder in crystalline powders – a review of analytical techniques and their
application. International Journal of Pharmaceutics, 1999. Vol. 179, pp. 141–158.
[124] Hancock, B. C. and Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharmaceutical Reseach,
2000. Vol. 17, pp. 397–404.
[125] Byrn, S., Pfeiffer, R., Ganey, M., Hoiberg, C., and Poochikian, G. Pharmaceutical solids: a strategic approach to regulatory
considerations. Pharmaceutical Research, 1995. Vol. 12, pp. 945–954.
[126] Broadhead, J., Rouan, S. K. E., and Rhodes, C. T. The spray drying of pharmaceuticals. Drug Development and Industrial
Pharmacy, 1992. Vol. 18, pp. 1169–1206.
[127] Kerc, J. and Srcic, S. Thermal analysis of glassy pharmaceuticals. Thermochimica Acta, 1995. Vol. 248, pp. 81–95.
[128] Yu, L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Advanced Drug Delivery Reviews,
2001. Vol. 48, pp. 27–42.
[129] Hancock, B. C. and Zografi, G. Characteristics and significance of the amorphous state in pharmaceutical systems. Journal
of Pharmaceutical Sciences, 1997. Vol. 86, pp. 1–12.
[130] Giron, D. Thermal Analysis, microcalorimetry and combined techniques for the study of pharmaceuticals. Journal of Thermal
Analysis and Calorimetry, 1999. Vol. 56, pp. 1285–1304.
[131] Phipps, M. A. and Mackin, L. A. Application of isothermal microcalorimetry in solid state drug development. Pharmaceutical
Science and Technology Today, 2000. Vol. 3, pp. 9–17.
[132] Vippagunta, S. R., Brittain, H. G., and Grant, D. J. W. Crystalline solids. Advanced Drug Delivery Reviews, 2001. Vol.
48, pp. 3–26.
[133] Ward, G. H. and Schultz, R. K. Process-induced crystallinity changes in albuterol sulfate and its effect on powder physical
stability. Pharmaceutical Research, 1995. Vol. 12, pp. 773–779.
[134] Buckton, G. Characterisation of small changes in the physical properties of powders of significance for dry powder inhaler
formulations. Advanced Drug Delivery Reviews, 1997. Vol. 26, pp. 17–27.
[135] Byrn, S. R., Xu, W., and Newman, A. W. Chemical reactivity in solid-state pharmaceuticals: formulation implications.
Advanced Drug Delivery Reviews, 2001. Vol. 48, pp. 115–136.
[136] Ahlneck, C. and Zografi, G. The molecular basis of moisture effects on the physical and chemical stability of drugs
in the solid state. International Journal of Pharmaceutics, 1990. Vol. 62, pp. 87–95.
[137] Shalaev, E. Y. and Zografi, G. How does residual water affect the solid-state degradation of drugs in the amorphous
state? Journal of Pharmaceutical Sciences, 1996. Vol. 85, pp. 1137–1141.
[138] Hancock, B. C. and Zografi, G. The relationship between the glass transition temperature and the water content of amorphous
pharmaceutical solids. Pharmaceutical Research, 1994. Vol. 11, pp. 471–477.
[139] Kontny, M. J., Grandolfi, G. P., and Zografi, G. Water vapor sorption of water soluble substances: Studies of crystalline
solids below their critical relative humidities. Pharmaceutical Research, 1987. Vol. 4, pp. 104–112.
[140] Fukuoka, E., Makita, M., and Yamamura, S. Glassy state of pharmaceuticals. III. Thermal properties and stability of
glassy pharmaceuticals and their binary glass systems. Chemical and Pharmaceutical Bulletin, 1989. Vol. 37, pp. 1047–1050.
[141] Timko, R. J. and Lordi, N. G. Thermal analysis studies of glass dispersion systems. Drug Development and Industrial
Pharmacy, 1984. Vol. 10, pp. 425–451.
[142] Nicholson, J. W. The Chemistry of Polymers. Cambridge, UK: The Royal Society of Chemistry, 1991. ISBN 0-85186-413-9
[143] Odian, G. Principles of Polymerization. New York, USA: John Wiley & Sons, Inc., 1991. ISBN 0-471-61020-8
[144] Leuner, C. and Dressman, J. Improving drug solubility for oral delivery using solid dispersions. European Journal of
Pharmaceutics and Biopharmaceutics, 2000. Vol. 50, pp. 47–60.
[145] Ford, J. L. The use of thermal analysis in the study of solid dispersions. Drug Development and Industrial Pharmacy,
1987. Vol. 13, pp. 1741–1777.
[146] Jenquin, M. R., Liebowitz, S. M., Sarabia, R. E., and McGinity, J. W. Physical and chemical factors influencing the
release of drugs from acrylic resin films. Journal of Pharmaceutical Sciences, 1990. Vol. 79, pp. 811–816.
[147] Jenquin, M. R. and McGinity, J. W. Characterization of acrylic resin matrix films and mechanisms of drug-polymer interactions.
International Journal of Pharmaceutics, 1994. Vol. 101, pp. 23–34.
[148] Lin, S. Y. and Perng, R. I. Solid-state interaction studies of drugs/polymers: I. Indomethacin/Eudragit E, RL or S resins.
STP Pharma Sciences, 1993. Vol. 3, pp. 465–471.
[149] Lin, S.-Y., Cheng, C.-L., and Perng, R.-I. Solid-state interaction studies of drug-polymers (II): Warfarin-Eudragit
E, RL, or S resins. European Journal of Pharmaceutical Sciences, 1994. Vol. 1, pp. 313–322.
[150] Lin, S.-Y., Liao, C.-M., Hsiue, G.-H., and Liang, R.-C. Study of a theophylline-Eudragit L mixture using a combined
system of microscopic Fourier-transform infrared spectroscopy and differential scanning calorimetry. Thermochimica Acta, 1995.
Vol. 245, pp. 153–166.
[151] Lovrecich, M., Nobile, F., Rubessa, F., and Zingone, G. Effect of ageing on the release of indomethacin from solid dispersions
with Eudragits. International Journal of Pharmaceutics, 1996. Vol. 131, pp. 247–255.
[152] Wu, C. and McGinity, J. W. Influence of ibuprofen as a solid-state plasticizer in Eudragit RS 30 D on the physicochemical
properties of coated beads. AAPS PharmSciTech, 2001. Vol. 2, pp. Article 24.
[153] Yüksel, N., Tincer, T., and Baykara, T. Interaction between nicardipine hydrochloride and polymeric microspheres for
a controlled release system. International Journal of Pharmaceutics, 1996. Vol. 140, pp. 145–154.
[154] Crowley, K. J. and Zografi, G. The effect of low concentrations of molecularly dispersed poly(vinylpyrrolidone) on indomethacin
crystallization from the amorphous state. Pharmaceutical Research, 2003. Vol. 20, pp. 1417–1422.
[155] Karjalainen, T., Rich, J., and Seppälä, J. Release of model compounds from modified lactone copolymers. Journal of Applied
Polymer Science, 2001. Vol. 81, pp. 2118–2126.
[156] Rich, J., Karjalainen, T., Ahjopalo, L., and Seppälä, J. Model compound release from DL-lactide/e-caprolactone copolymers
and evaluation of specific interactions by molecular modeling. Journal of Applied Polymer Science, 2002. Vol. 86, pp. 1–9.
[157] Pignatello, R., Amico, D., Chiechio, S., Spadaro, C., and Puglisi, G. Preparation and analgesic activity of Eudragit
RS100 microparticles containing diflunisal. Drug Delivery, 2001. Vol. 8, pp. 35–45.
[158] Pignatello, R., Ferro, M., and Puglisi, G. Preparation of solid dispersions of nonsteroidal anti-inflammatory drugs
with acrylic polymers and studies on mechanisms of drug-polymer interactions. AAPS PharmSciTech, 2002. Vol. 3, pp. Article
10.
[159] Wu, C. and McGinity, J. W. Non-traditional plasticization of polymeric films. International Journal of Pharmaceutics,
1999. Vol. 177, pp. 15–27.
[160] Mura, P., Manderioli, A., Bramanti, G., and Ceccarelli, L. Properties of solid dispersions of naproxen in various polyethylene
glycols. Drug Development and Industrial Pharmacy, 1996. Vol. 22, pp. 909–916.
[161] Shukla, A. J. Polymethacrylates. In: Wade, A. and Weller, P. J. (Eds.) Handbook of Pharmaceutical Excipients. Washington
DC, USA: American Pharmaceutical Association, Pharmaceutical Press, 1994. ISBN 0-91730-66-8.
[162] Dittgen, M., Durrani, M., and Lehmann, K. Acrylic polymers A review of pharmaceutical applications. STP Pharma Sciences,
1997. Vol. 7, pp. 403–437.
[163] El-Kamel, A. H., Sokar, M. S., Al Gamal, S. S., and Naggar, V. F. Preparation and evaluation of ketoprofen floating
oral delivery system. International Journal of Pharmaceutics, 2001. Vol. 220, pp. 13–21.
[164] Esposito, E., Roncarati, R., Cortesi, R., Cervellati, F., and Nastruzzi, C. Production of Eudragit microparticles by
spray-drying technique: influence of experimental parameters on morphological and dimensional characteristics. Pharmaceutical
Development and Technology, 2000. Vol. 5, pp. 267–278.
[165] Esposito, E., Cervellati, F., Menegatti, E., Nastruzzi, C., and Cortesi, R. Spray dried Eudragit microparticles as encapsulation
devices for vitamin C. International Journal of Pharmaceutics, 2002. Vol. 242, pp. 329–334.
[166] Palmieri, G. F., Wehrlé, P., and Stamm, A. Evaluation of spray-drying as a method to prepare microparticles for controlled
drug release. Drug Development and Industrial Pharmacy, 1994. Vol. 20, pp. 2859–2879.
[167] Takeuchi, H., Handa, T., and Kawashima, Y. Controlled release teophylline tablet with acrylic polymers prepared by spray-drying
technique in aqueous system. Drug Development and Industrial Pharmacy, 1989. Vol. 15, pp. 1999–2016.
[168] Al Khouri Fallouh, N., Roblot-Treupel, L., Fessi, H., Devissaguet, J. P., and Puisieux, F. Development of a new process
for the manufacture of polyisobutylcyanoacrylate nanocapsules. International Journal of Pharmaceutics, 1986. Vol. 28, pp.
125–132.
[169] Rollot, J. M., Couvreur, P., Roblot-Treubel, L., and Puisieux, F. Physicochemical and morphological characterization
of polyisobutyl cyanoacrylate nanocapsules. Journal of Pharmaceutical Sciences, 1986. Vol. 75, pp. 361–364.
[170] Fessi, H., Puisieux, F., Devissaguet, J. P., Ammoury, N., and Benita, S. Nanocapsule formation by interfacial polymer
deposition following solvent displacement. International Journal of Pharmaceutics, 1989. Vol. 55, pp. R1–R4.
[171] Magenheim, B. and Benita, S. Nanoparticle characterization: a comprehensive physicochemical approach. STP Pharma Sciences,
1991. Vol. 1, pp. 221–241.
[172] Arshady, R. Preparation of biodegradable microspheres and microcapsules: 2. Polylactides and related polyesters. Journal
of Controlled Release, 1991. Vol. 17, pp. 1–22.
[173] Bodmeier, R., Chen, H., Tyle, P., and Jarosz, P. Spontaneous formation of drug-containing acrylic nanoparticles. Journal
of Microencapsulation, 1991. Vol. 8, pp. 161–170.
[174] Kawashima, Y., Niwa, T., Handa, T., Takeuchi, H., Iwamoto, T., and Itoh, K. Preparation of controlled-release microspheres
of ibuprofen with acrylic polymers by a novel quasi-emulsion solvent diffusion method. Journal of Pharmaceutical Sciences,
1989. Vol. 78, pp. 68–72.
[175] Kawashima, Y., Iwamoto, T., Niwa, T., Takeuchi, H., and Hino, T. Size control of ibuprofen microspheres with an acrylic
polymer by changing the pH in an aqueous dispersion medium and its mechanism. Chemical and Pharmaceutical Bulletin, 1993.
Vol. 41, pp. 191–195.
[176] Quintanar-Guerrero, D., Allémann, E., Doelker, E., and Fessi, H. Preparation and characterization of nanocapsules from
preformed polymers by a new process based on emulsification-diffusion technique. Pharmaceutical Research, 1998. Vol. 15, pp.
1056–1062.
[177] Peltonen, L., Koistinen, P., Karjalainen, M., Häkkinen, A., and Hirvonen, J. The effect of cosolvents on the formulation
of nanoparticles from low-molecular-weight poly(l)lactide. AAPS PharmSciTech, 2002. Vol. 3, pp. Article 32.
[178] Bodmeier, R., Wang, H., Dixon, D. J., Mawson, S., and Johnston, K. P. Polymeric microspheres prepared by spraying into
compressed carbon dioxide. Pharmaceutical Research, 1995. Vol. 12, pp. 1211–1217.
[179] Bodmeier, R. and Chen, H. Preparation and characterization of microspheres containing the anti-inflammatory agents,
indomethacin, ibuprofen, and ketoprofen. Journal of Controlled Release, 1989. Vol. 10, pp. 167–175.
[180] Bodmeier, R. and McGinity, J. W. Solvent selection in the preparation of poly(DL-lactide) microspheres prepared by the
solvent evaporation method. International Journal of Pharmaceutics, 1988. Vol. 43, pp. 179–186.
[181] Jain, R. A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials, 2000. Vol. 21, pp. 2475–2490.
[182] Müller, C. R., Schaffazick, S. R., Pohlmann, A. R., de Lucca Freitas, L., Pesce da Silveira, N., Dalla Costa, T., and
Guterres, S. S. Spray-dried diclofenac-loaded poly(e-caprolactone) nanocapsules and nanospheres. Preparation and physicochemical characterization. Pharmazie, 2001. Vol. 56, pp.
864–867.
[183] Vergote, G. J., Vervaet, C., Van Driessche, I., Hoste, S., De Smedt, S., Demeester, J., Jain, R. A., Ruddy, S., and
Remon, J. P. An oral controlled release matrix pellet formulation containing nanocrystalline ketoprofen. International Journal
of Pharmaceutics, 2001. Vol. 219, pp. 81–87.
[184] De Jaeghere, F., Allémann, E., Leroux, J.-C., Stevels, W., Feijen, J., Doelker, E., and Gurny, R. Formulation and lyoprotection
of poly(lactic acid-co-ethylene oxide) nanoparticles: influence on physical stability and in vitro cell uptake. Pharmaceutical Research, 1999. Vol. 16, pp. 859–866.
[185] Reverchon, E. Supercritical antisolvent precipitation of micro- and nano-particles. Journal of Supercritical Fluids,
1999. Vol. 15, pp. 1–21.
[186] Reverchon, E. and Della Porta, G. Production of antibiotic micro- and nano-particles by supercritical antisolvent precipitation.
Powder Technology, 1999. Vol. 106, pp. 23–29.
[187] Reverchon, E., De Marco, I., and Della Porta, G. Rifampicin micoparticles production by supercritical antisolvent precipitation.
International Journal of Pharmaceutics, 2002. Vol. 243, pp. 83–91.
[188] Rogers, T. L., Johnston, K. P., and Williams III, R. O. Solution-based particle formation of pharmaceutical powders
by supercritical or compressed fluid CO2 and cryogenic spray-freezing technologies. Drug Development and Industrial Pharmacy, 2001. Vol. 27, pp. 1003–1015.
[189] Tom, J. W. and Debenedetti, P. G. Particle formation with supercritical fluids – a review. Journal of Aerosol Science,
1991. Vol. 22, pp. 555–584.
[190] Tu, L. S., Dehghani, F., and Forster, N. R. Micronisation and microencapsulation of pharmaceuticals using a carbon dioxide
antisolvent. Powder Technology, 2002. Vol. 126, pp. 134–149.
[191] Yeo, S.-D., Lim, G.-B., Debenedetti, P. G., and Bernstein, H. Formation of microparticulate protein powders using a
supercritical fluid antisolvent. Biotechnology and Bioengineering, 1993. Vol. 41, pp. 341–346.
[192] York, P. Strategies for particle desing using supercritical fluid technologies. Pharmaceutical Science & Technology
Today, 1999. Vol. 2, pp. 430–440.
[193] Palakodaty, S., York, P., and Pritchard, J. Supercritical fluid processing of materials from aqueous solutions: The
application of SEDS to lactose as a model substance. Pharmaceutical Research, 1998. Vol. 15, pp. 1835–1843.
[194] Moshashaée, S., Bisrat, M., Forbes, R. T., Nyqvist, H., and York, P. Supercritical fluid processing of proteins I: Lysozyme
precipitation from organic solution. European Journal of Pharmaceutical Sciences, 2000. Vol. 11, pp. 239–245.
[195] Palakodaty, S. and York, P. Phase behavioural effects on particle formation processes using supercritical fluids. Pharmaceutical
research, 1999. Vol. 16, pp. 976–985.
[196] Juppo, A. M., Boissier, C., and Khoo, C. Evaluation of solid dispersion particles prepared with SEDS. International
Journal of Pharmaceutics, 2003. Vol. 250, pp. 385–401.
[197] Giunchedi, P. and Conte, U. Spray-drying as a preparation method of microparticulate drug delivery system: an overview.
STP Pharma Sciences, 1995. Vol. 5, pp. 276–290.
[198] Nielsen, F. Spray drying pharmaceuticals. Manufacturing Chemist, 1982. Vol. 57, pp. 38–41.
[199] Bodmeier, R. and Chen, H. Preparation of biodegradable poly(+)lactide microparticles using a spray-drying technique. Journal of Pharmacy and Pharmacology, 1988. Vol. 40, pp. 754–757.
[200] Wang, F.-J. and Wang, C.-H. Sustained release of etanidazole from spray dried microspheres prepared by non-halogenated
solvents. Journal of Controlled Release, 2002. Vol. 81, pp. 263–280.
[201] Pignatello, R., Vandelli, M. A., Giunchedi, P., and Puglisi, G. Properties of tolmetin-loaded Eudragit RL100 and Eudragit
RS100 microparticles prepared by different techniques. STP Pharma Sciences, 1997. Vol. 7, pp. 148–157.
[202] Maa, Y.-F., Nguyen, P.-A. T., and Hsu, S. W. Spray-drying of air-liquid interface sensitive recombinant human growth
hormone. Journal of Pharmaceutical Sciences, 1998. Vol. 87, pp. 152–159.
[203] Jung, J.-Y., Yoo, S. D., Lee, S.-H., Kim, K.-H., Yoon, D.-S., and Lee, K.-H. Enhanced solubility and dissolution rate
of itraconazole by a solid dispersion technique. International Journal of Pharmaceutics, 1999. Vol. 187, pp. 209–218.
[204] Corrigan, O. I., Holohan, E. M., and Reilly, M. R. Physicochemical properties of indomethacin and related compounds
co-spray dried with polyvinylpyrrolidone. Drug Development and Industrial Pharmacy, 1985. Vol. 11, pp. 677–695.
[205] Kristmundsdóttir, T., Gudmundsson, Ó. S., and Ingvarsdóttir, K. Release of diltiazem from Eudragit microparticles prepared
by spray-drying. International Journal of Pharmaceutics, 1996. Vol. 137, pp. 159–165.
[206] Takenaka, H., Kawashima, Y., and Lin, S. Y. Polymorphism of spray-dried microencapsulated sulfamethoxazole with cellulose
acetate phtalate and colloidal silica, montmorillonite, or talc. Journal of Pharmaceutical Sciences, 1981. Vol. 70, pp. 1256–1260.
[207] Tsapis, N., Bennett, D., Jackson, B., Weitz, D. A., and Edwards, D. A. Trojan particles: Large porous carriers of nanoparticles
for drug delivery. Proceedings of the National Academy of Sciences of the United States of America, 2002. Vol. 99, pp. 12001–12005.
[208] Wan, L. S. C., Heng, P. W. S., and Chia, C. G. H. Preparation of coated particles using a spray drying process with
an aqueous system. International Journal of Pharmaceutics, 1991. Vol. 77, pp. 183–191.
[209] Wan, L. S. C., Heng, P. W. S., and Chia, C. G. H. Plasticizers and their effects on microencapsulation process by spray-drying
in an aqueous system. Journal of Microencapsulation, 1992. Vol. 9, pp. 53–62.
[210] Wan, L. S. C., Heng, P. W. S., and Chia, C. G. H. Spray drying as a process for microencapsulation and the effect of
different coating polymers. Drug Development and Industrial Pharmacy, 1992. Vol. 18, pp. 997–1011.
[211] Watanabe, W., Ahonen, P., Kauppinen, E., Brown, D., Jokiniemi, J., and Muttonen, E. (2003). “Inhalation Particles.” Patent WO 03/002111, Orion Corporation.
[212] TSI Incorporated. Model 3075/3076 constant output atomizer instruction manual. St. Paul, USA: TSI Incorporated, 2000.
[213] Brown, D. P. Development of a three-dimensional coupled flow, species and aerosol model: Applications to particle deposition
in gas turbines and aerosol formation and growth in jet engine exhausts. Ph.D. thesis. Cincinnati, USA: University of Cincinnati,
Environmental Engineering, 1996.
[214] Brown, D. P., Jokiniemi, J. K., and Kauppinen, E. I. Heat and mass transfer in aerosol systems. Proceedings of Vapor
Phase Synthesis of Materials III, Haikko, Finland, July 18–23, 1999.
[215] Hillamo, R. E. and Kauppinen, E. I. On the performance of the Berner low pressure impactor. Aerosol Science and Technology,
1991. Vol. 14, pp. 33–47.
[216] Giunchedi, P., Conti, B., Maggi, L., and Conte, U. Cellulose acetate butyrate and polycaprolactone for ketoprofen spray-dried
microsphere preparation. Journal of Microencapsulation, 1994. Vol. 11, pp. 381–393.
[217] Moretti, M. D. L., Gavini, E., Juliano, C., Pirisino, G., and Giunchedi, P. Spray-dried microspheres containing ketoprofen
formulated into capsules and tablets. Journal of Microencapsulation, 2001. Vol. 18, pp. 111–121.
[218] Palmieri, G. F., Bonacucina, G., Di Martino, P., and Martelli, S. Gastro-resistant microspheres containing ketoprofen.
Journal of Microencapsulation, 2002. Vol. 19, pp. 111–119.
[219] Palmieri, G. F., Elisei, I., Di Martino, P., and Martelli, S. Formulation of microparticulate systems for modified release
containing ketoprofen. 19th Pharmaceutical Technology Conference, April 11.–13., Italy, 2000.
[220] Sveinsson, S. J. and Kristmundsdóttir, T. Naproxen microcapsules: preparation and in vitro characterization. International
Journal of Pharmaceutics, 1992. Vol. 82, pp. 129–133.
[221] Yamada, T., Onishi, H., and Machida, Y. Sustained release ketoprofen microparticles with ethylcellulose and carboxymethylethylcellulose.
Journal of Controlled Release, 2001. Vol. 75, pp. 271–282.
[222] Kim, J.-H., Paxton, T. E., and Tomasko, D. L. Microencapsulation of naproxen using rapid expansion of supercritical
solutions. Biotecnology Progress, 1996. Vol. 12, pp. 650–661.
[223] Rohdewald, P. Beclometasondipropionat. In: Hartke, K. (Ed.) Kommentar zum Europäischen Arzneibuch. Stuttgart, Eschborn,
Germany: Wissenschaftliche Verlagsgesellschaft mbH, Govi-Verlag, 2001. ISBN 3-8047-1791-8.
[224] Avdeef, A., Berger, C. M., and Brownell, C. pH-metric solubility. 2: correlation between the acid-base titration and
the saturation shake-flask solubility-pH methods. Pharmaceutical Research, 2000. Vol. 17, pp. 85–89.
[225] Yalkowsky, S. H. and Dannenfelser, R. M. The Aquasol Database of Aqueous Solubility. Tucson, USA: College of Pharmacy,
University of Arizona, 1992.
[226] Millard, J. W. and Myrdal, P. B. Anhydrous beclomethasone dipropionate. Acta Crystallographica E, 2002. Vol. 58.
[227] Briard, P. P. and Rossi, J. C. Kétoprofène. Acta Crystallographica, 1990. Vol. C46, pp. 1036–1038.
[228] Ravikumar, K., Rajan, S. S., and Pattabhi, V. Structure of naproxen. Acta Crystallographica C, 1985. Vol. 41, pp. 280–282.
[229] Greenley, R. Z. Q and e values for Free Radical Copolymerization of Vinyl Monomers and Telogens. In: Brandrup, J. and Immergut, E. H. (Eds.) Polymer Handbook.
pp. II/267–II/274. New York, USA: John Wiley & Sons, Inc., 1989. ISBN 0-471-81244-7.
[230] Porsch, B., Hillang, I., Karlsson, A., and Sundelöf, L.-O. Ion-exclusion controlled size-exclusion chromatography of
methacryllic acid-methyl methacrylate copolymers. Journal of Chromatography A, 2000. Vol. 872, pp. 91–99.
[231] Wittgren, B., Welinder, A., and Porsch, B. Molar mass characterization of cationic methyl methacrylate – ethyl acrylate
copolymers using size-exclusion chromatography with online multi-angle light scattering and refractometric detection. Journal
of Chromatography A, 2003. Vol. 1002, pp. 101–109.
[232] Atkins, P. W. Physical Chemistry. Oxford, UK: Oxford University Press, 1990. ISBN 0-19-855283-1.
[233] Neubeck, M. Ketoprofen. In: Hartke, K. (Ed.) Kommentar zum Europäischen Arzneibuch. Stuttgart, Eschborn, Germany: Wissenschaftliche
Verlagsgesellschaft mbH, Govi-Verlag, 2001. ISBN 3-8047-1791-8.
[234] Wirbizki, E. Naproxen. In: Hartke, K. (Ed.) Kommentar zum Europäischen Arzneibuch. Stuttgart, Eschborn, Germany: Wissenschaftliche
Verlagsgesellschaft mbH, Govi-Verlag, 2001. ISBN 3-8047-1791-8.
[235] United States Pharmacopeia 25. <724> Drug Release. Rockville, MD, USA: United States Pharmacopeial Convention, 2001. ISBN 1-889788-10-4.
[236] Gurav, A., Kodas, T., Pluym, T., and Xiong, Y. Aerosol Processing of Materials. Aerosol Science and Technology, 1993.
Vol. 19, pp. 411–452.
[237] Lefebvre, A. H. Atomization and Sprays. In: Chigier, N. (Ed.) Combustion: An International Series. New York, USA: Hemisphere
Publishing Corporation, 1989. ISBN 0-89116-603-3.
[238] Che, S., Sakurai, O., Shinozaki, K., and Mizutami, N. Particle structure control through intraparticle reactions by
spray pyrolysis. Journal of Aerosol Science, 1998. Vol. 29, pp. 271–278.
[239] Zhou, X. D., Zhang, S. C., Huebner, W., Ownby, P. D., and Gu, H. Effect of the solvent on the particle morphology of
spray dried PMMA. Journal of Materials Science, 2001. Vol. 36, pp. 3759–3768.
[240] Jayanthi, G. V., Zhang, S. C., and Messing, G. L. Modeling of Solid Particle Formation During Solution Aerosol Thermolysis.
The Evaporation Stage. Aerosol Science and Technology, 1993. Vol. 19, pp. 478–490.
[241] Xiong, Y. and Kodas, T. T. Droplet evaporation and solute precipitation during spray pyrolysis. Journal of Aerosol Science,
1993. Vol. 24, pp. 893–908.
[242] Leong, K. H. Morphological control of particles generated from the evaporation of solution droplets: theoretical considerations.
Journal of Aerosol Science, 1987. Vol. 18, pp. 511–524.
[243] Giunchedi, P., Torre, M. L., Maggi, L., Conti, B., and Conte, U. Cellulose acetate trimetillate ethylcellulose blends
for non-steroidal anti-inflammatory drug (NSAID) microspheres. Journal of Microencapsulation, 1996. Vol. 13, pp. 89–98.
[244] Leong, K. H. Morphological control of particles generated from the evaporation of solution droplets: experiment. Journal
of Aerosol Science, 1987. Vol. 18, pp. 525–552.
[245] Dubernet, C. Thermoanalysis of microspheres. Thermochimica Acta, 1995. Vol. 248, pp. 259–269.
[246] Dubernet, C., Rouland, J. C., and Benoit, J. P. Ibuprofen-loaded ethylcellulose microspheres: analysis of the matrix
structure by thermal analysis. Journal of Pharmaceutical Sciences, 1991. Vol. 80, pp. 1029–1033.
[247] Wunderlich, B. Thermal Analysis. San Diego, USA: Academic Press, Inc., 1990. ISBN 0-12-765605-7.
[248] Morimoto, Y., Kokubo, T., and Sugibayashi, K. Diffusion of drugs in acrylic-type pressure-sensitive adhesive matrix.
II. Influence of interaction. Journal of Controlled Release, 1992. Vol. 18, pp. 113–122.
[249] Saleem, M., Asfour, A.-F. A., and De Kee, D. Diffusion of organic penetrants through low density polyethylene (LDPE)
films: effect of size and shape of the penetrant molecules. Journal of Applied Polymer Science, 1989. Vol. 37, pp. 617–625.
[250] Lin, S.-Y., Chen, K.-S., and Run-Chu, L. Organic esters of plasticizers affecting the water absorption, adhesive property,
glass transition temperature and plasticizer permanence of Eudragit acrylic films. Journal of Controlled Release, 2000. Vol.
68, pp. 343–350.
[251] Cilurzo, F., Minghetti, P., Casiraghi, A., and Montanari, L. Characterization of nifedipine solid dispersions. International
Journal of Pharmaceutics, 2002. Vol. 242, pp. 313–317.
[252] Wu, C. and McGinity, J. W. Influence of methylparaben as a solid-state plasticizer on the physicochemical properties
of Eudragit RS PO hot-melt extrudates. European Journal of Pharmaceutics and Biopharmaceutics, 2003. Vol. 56, pp. 95–100.
[253] Mura, P., Faucci, M. T., Parrini, P. L., Furlanetto, S., and Pinzauti, S. Influence of the preparation method on the
physicochemical properties of ketoprofen-cyclodextrin binary systems. International Journal of Pharmaceutics, 1999. Vol. 179,
pp. 117–128.
[254] Sancin, P., Caputo, O., Cavallari, C., Passerini, N., Rodriguez, L., Cini, M., and Fini, A. Effects of ultrasound-assisted
compaction on Ketoprofen/Eudragit S100 mixtures. European Journal of Pharmaceutical Sciences, 1999. Vol. 7, pp. 207–213.
[255] Röhm Pharma Polymers Degussa. Specifications and test methods for Eudragit. Darmstadt, Germany: Röhm GmbH & Co. KG,
2003.
[256] Lin, S.-Y., Yu, H.-L., and Li, M.-J. Formation of six-membered cyclic anhydrides by thermally induced intramolecular
ester condensation in Eudragit E film. Polymer, 1999. Vol. 40, pp. 3589–3593.
[257] Morawetz, H. Theoretical aspects of chain configuration and counterion distribution in solutions of flexible chain polyelectrolytes.
In: S. A. Rice and M. Nagasawa, Eds. Polyelectrolyte Solutions. pp. 207–248. New York: Academic Press, 1961.
[258] Tanford, C. Physical Chemistry of Macromolecules. New York: John Wiley & Sons, Inc., 1961. ISBN 0-471-84447-0.
[259] Meares, P. Transport in Ion-exchange Polymers. In: Crank, J. and Park,
G. S. (Eds.) Diffusion in Polymers. pp. 373–428. London: John Wright & Sons, Inc., 1968.
[260] Higuchi, T. Mechanism of sustained-action medication. Journal of Pharmaceutical Sciences, 1963. Vol. 52, pp. 1145–1149.
[261] Corrigan, O. I. Mechanisms of dissolution of fast release solid dispersions. Drug Development and Industrial Pharmacy,
1985. Vol. 11, pp. 697–724.
[262] Lennernäs, H., Knutson, L., Knutson, T., Lesko, L., Salmonson, T., and Amidon, G. L. Human effective permeability data
for furosemide, hydrochlortiazide, ketoprofen, and naproxen to be used in the proposed biopharmaceutical classification for
IR-products. Pharmaceutical research, 1995. Vol. 12, pp. S-396.
[263] Pharmaca Fennica. Helsinki: Lääketietokeskus Oy, 2004. ISBN 0355-7472.
[264] Yu, L. X., Crison, J. R., and Amidon, G. L. Compartmental transit and dispersion model analysis of small intestinal
transit flow in humans. International Journal of Pharmaceutics, 1996. Vol. 140, pp. 111–118.
[265] Yu, L. X. and Amidon, G. L. A compartmental absorption and transit model for estimating oral drug absorption. International
Journal of Pharmaceutics, 1999. Vol. 186, pp. 199–125.
[266] Jamali, F. and Brocks, D. R. Clinical pharmacokinetics of ketoprofen and its enantiomers. Clinical Pharmacokinetics,
1990. Vol. 19, pp. 197–217.
|